An important peer-reviewed study that provided concrete information about the “excess risk” of negative side effects from Pfizer-BioNTech and Moderna mRNA vaccines in a separate “randomized clinical trial” looks to be the first of its type.
The findings of the widely regarded scientific study support the validity of the many patients’ worries with mRNA vaccinations.
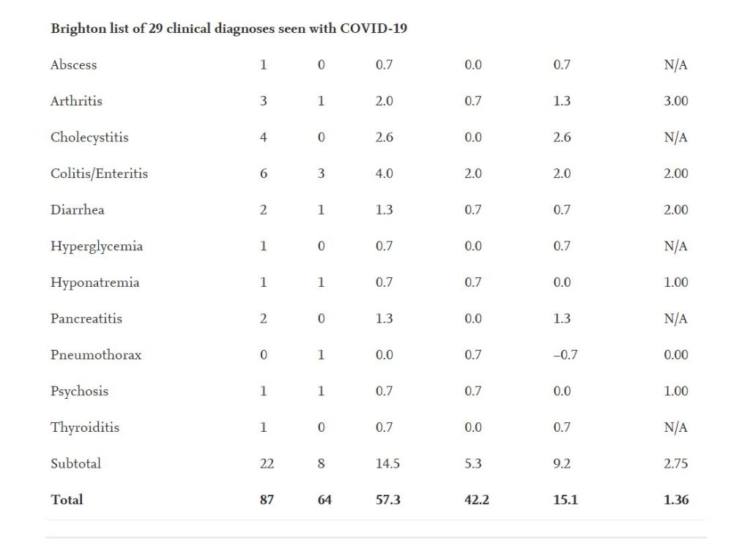
The extra risk of significant AESIs was larger in the Moderna trial (15.1 per 10,000 participants) than the risk reduction for COVID-19 hospitalization in comparison to the placebo group (6.4 per 10,000 participants), the research revealed.
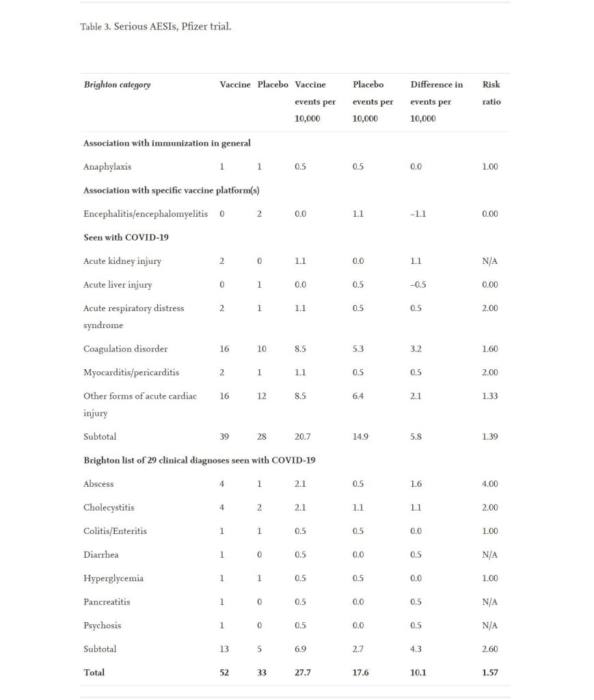
The extra risk of significant AESIs in the Pfizer trial (10.1 per 10,000) was higher than the risk reduction for COVID-19 hospitalization in comparison to the placebo group (2.3 per 10,000 individuals), according to the report.
On August 31, 2022, the study was posted on ScienceDirect. Researchers from Stanford University, the University of Maryland, and UCLA are among the authors. The study presents the following list of the respective mRNA vaccines’ confirmed adverse events (or side effects). Additionally, it provides risk comparisons to Covid-19 (over 1 is a factor increase, under 1 is a factor decrease). The Pfizer list is as follows:
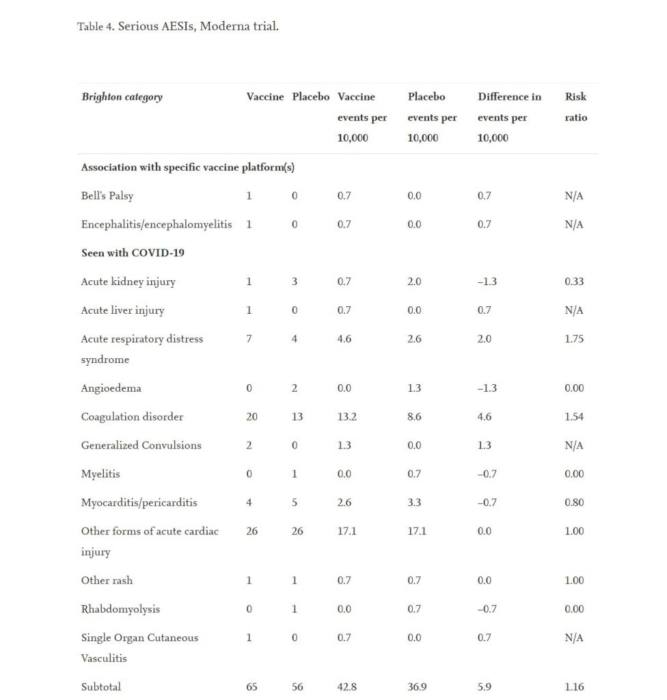
The study also shows known complications for Covid-19.
The authors write that whereas randomized trials provide strong evidence for assessing causal effects, the scarcity of such data forces harm-benefit evaluations to additionally take observational research into account. “Hundreds of millions of doses of Pfizer and Moderna COVID-19 vaccines have been delivered since their emergency authorization in December 2020, and post-authorization observational data offer a complementary chance to research AESIs. Cohort studies (which use medical claims or electronic health records) and disproportionality analysis (which use spontaneous adverse event reporting systems) are two examples of post-authorization observational safety studies.
Rephrased from Becker News